What This Book Offers Instructors
Philosophy
Angela Duckworth’s book Grit: The Power of Passion and Perseverance makes excellent points about
the psychology of teaching and learning. One of those points is that focused, targeted practice time is
essential.
For sophomore organic chemistry maybe 40 – 50 hours of that type of practice per semester is enough
for a student to get a good grade, provided it is really focused and targeted. The key issue is how to
guide students to focus on, and to target parts of the course that have the most impact on their
understanding. Trying to memorize a textbook or notes from a textbook, is certainly not optimal.
This is a workbook, not a textbook, for students to practice essential concepts in the first semester of
sophomore organic chemistry. Book 2 in this series covers the second semester.
This first book is organized by concepts (eg mechanisms), not by functional groups, but nevertheless
follows roughly the same sequence as chemistry textbooks like those by McMurry and Wade. It has
features many instructors might recommend for quality study time. It targets key concepts that can be
applied repeatedly and avoid facts that students might think are important to memorize but are not.
Throughout, students are encouraged to research factual information that is easily understood;
instructors do not have to teach this. Twenty-first-century teenagers do not like to be lectured, especially about the simple and obvious material, but they have no hesitation whipping out their devices to research things for themselves.
Students need to compare their answers against model solutions. Consequently, answers for this
the workbook is available via the By Inquisition website (www.byinquisition.org) and will be available in
some videos online.
Uses Of This Book
Strategies
• Motivated students can use this text to supplement what they are being taught in sophomore
organic chemistry; and,
• instructors implementing “flipped” paradigms may use this book as a template to solve some of
the problems in class, and leave others for the students to work off-site.
Flipping Organic Chemistry Classes
About a decade ago, I decided to lecture less and focus more on teaching students strategies to learn
organic chemistry. Before that I dutifully covered syllabi by presenting material from the textbook,
expecting students to copy and learn it. I thought that simply showing vast amounts of information would inspire students to learn it. However, when shown a table of functional groups, for instance, most
students did not learn most of them until it was far too late. People would emerge from my lectures with
only a few memories, and a poor replication of a textbook, containing errors from my lectures and their
copying.
Now I teach sophomore organic from a collection of problems that have evolved into these workbooks. In class, I introduce concepts colloquially asking questions as I go, and ruthlessly calling students by name.
In that way, about 25 % of the problems in these books are solved on-site. In the next lecture, there will
be a quiz on that material including problems I did not solve. Most of the exams and the final also are
based on any of the problems distributed. Outside class, students may ask me about concepts, but not
answers to specific problems.
The strategy described above “flips” a class: placing the expectations on students to understand material outside of class, while the contact time is focused on solving problems involving new concepts. I enjoy flipped teaching more than lecturing, and overall the students seem to respond better.
Book Structure
Each section in this book is designed to be the focus of one 75 min class period, ie ideally about 10 – 15
pages of content. Thus the book is divided into 23 sections, which approximately corresponds to a
semester of instruction, with a few class time slots for review and exams. Listening to anyone for 75 min
is tough, particularly me, but the quiz breaks the routine and after that, I show chemistry videos from the web or demonstrations. If I do not finish all the intended problems in any given lecture, the rest of the questions automatically become homework.
This workbook could be used with any decent organic chemistry textbook, including supposedly out-of-date editions obtained cheaply via re-sale. I have tried to make the book attractive but affordable. It is intended to be light enough to carry to class and has space for students to write in answers and keep
they organized; they do not necessarily have to bring paper.
Most new textbooks now are sold as a package wherein students pay a lot for online problems, and only a little more for the text; this suits publishers because online subscriptions have no resale value.
Instructors like the arrangement too for the pedagogical value, and because all the problems are graded automatically. However, it is expensive for the students, and sometimes the online problems are not ideal.
These workbooks have no online learning component, and I appreciate that many instructors want one.
For that reason, I suggest students be asked to buy the online component from Sapling Learning
(http://www2.saplinglearning.com). Sapling Learning can provide a set of problems earmarked for most
sections. To learn more about using Sapling Learning, go to www.meetme.so/SaplingLearning or email
support@saplinglearning.com. Combinations of this book, older editions of a textbook, and an online
account from Sapling Learning can be more affordable than a conventional textbook/online learning
bundle.
Content
Reactions With Parallels In Vivo
Approximately 1800 people take sophomore organic chemistry each semester at the university where I
teach, but each year less than 100 students graduate as chemistry majors. More than 90% of those
1800 students major in other subjects relating to the biological sciences. I believe we should teach to
that 90 % and material that exclusively relates to laboratory organic syntheses tends to complicate and
confuse. My preference is that methods for laboratory organic syntheses (a topic I love) should be taught in upper-level classes after chemistry that has parallels in vivo is covered at the sophomore level.
This workbook focuses on chemistry useful to students majoring in biomedical sciences (including
chemistry majors). Reactions exclusively for synthetic organic chemistry are not emphasized, though
some are included because many instructors want it. Thus, mercuration, hydroboration, organometallic
cross-couplings, alkene metathesis, and regioselective serial additions of electrophiles to benzene
derivatives, and free radical halogenations are not included.
Fundamental Concepts
Like nearly all books for undergraduate chemistry, this one introduces hybridization early, but it also
trains students to identify hybridization states of atoms in larger molecules relevant to medicine or
biochemistry. Section 2 introduces minimalist molecular structure representations (ie usually not
involving the symbols C or H), and leads students to realize zigzag conformations are favored because of preferred conformations of acyclic hydrocarbons. Thereafter, minimalist representations of organic
molecules are used throughout.
Chemists, in general, tend to use various abbreviations for fragments and to represent functional groups in different ways; this makes learning organic chemistry particularly hard for the students. To help, section
3 of this book introduces functional groups. Effort that might have been spent on classical nomenclature
is spent on introducing the ways functional groups are drawn because it is more important to distinguish
an ester and an amide than it is to be able to name 3-methylhexane and not use “4-methylhexane”.
3 Section 5 of this book is completely devoted to electron flow. This is one of the most difficult skills to
teach, but one of the most important to learn. Consequently, this section is followed by another one (6)
on curly arrows applied to resonance structures.
Students who solve sections 1 – 6 without consulting the model answers will be in pole positions for the
rest of the course; these sections are the most important in this two-book series. The rest of the content is more conventional and self-explanatory. There is one section on cycloadditions (16) particularly azidealkyne reactions that often feature in biological chemistry, grouped with other cycloadditions that can have applications in biomedicinal chemistry.
Unlike most classical textbooks for sophomore organic, there is some discussion on fundamentals of
fluorescence and to introduce a few common fluors (after UV in section 19). This is included because of
the ubiquitous applications of fluorescent probes in the biological sciences. However, there is little
material covered in Book 1 that would not be found in classical organic textbooks for US sophomore
organic chemistry.
What This Book Offers Students
Philosophy
For sophomore organic chemistry, 40 – 50 hours of
focused, targeted practice per semester maybe
enough to get a good grade. The catch is that it
must be focused study targeted on the parts of the
a course that you do not already understand.
Focused means no distractions (texts, emails,
calls, TV, casual web browsing, etc). Targeted is
harder to define, but attempts to memorize
particular sections of textbooks or lecture notes are
boring leads to loss of focus and is therefore
ineffective.
This workbook is intended to be a guided approach
to targeted study. It is mostly organized by
mechanisms, not by functional groups, because
recognition of similarities in related key concepts
making them easier to learn. Throughout, this
workbook avoids details that might seem important
to remember, but are not. Not everything is
covered from any particular sophomore organic
chemistry text (eg the one your instructor may
recommend) but, on the other hand, diligently
working through the problems in this book will be
helpful for any sophomore organic class taught
from any standard textbook.
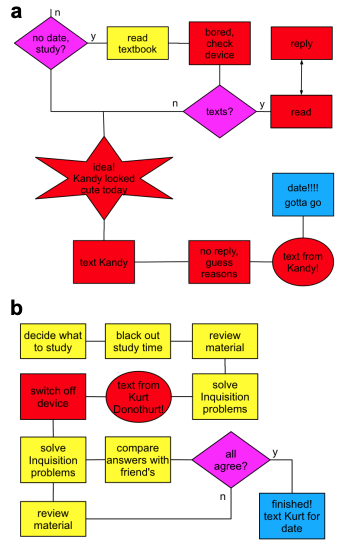
Figure 1. a Student Kurt’s studies based on a conventional
the textbook is boring and untargeted, so he is open to
distractions; but, b Kandy is focused and targeted because
she is researching and reasoning answers herself, responding
to questions in a By Inquisition book.
How To Use This Book
(i) Gain a basic understanding of the material.
(ii) Attempt the problems without looking at the ideal answers provided on the website for this book.
(iii) When unable to solve a problem, determine if it is searching for a fact or testing understanding of a key concept.
(iv) If a problem requires memorization of a fact, but that memory is not available, look up the answer in the text, from the web, anywhere except the ideal answers provided on the website for this book.
(v) If a problem requires the application of a concept but the required understanding is not there yet, learn more about the concept, then try again without looking at the ideal answers on the website for this book.
(vi) Crosscheck your answers with friends, and discuss if necessary.
(vii) Finally, check the ideal solutions provided on the web if there is any uncertainty about the correct ones.
Understanding [as in (i)] can be gained by targeted web surfing, from a textbook, going to lectures,
talking to friends or instructors, or watching appropriate videos from sources like YouTube. The best way to gain understanding is to do all these things.